Journal article
Densities, Excess and Partial Molar Volumes of (DFM + n-pentane or n-hexane or n-heptane or n-octane) Binary Mixtures at (T = 293.15, 298.15 and 303.15) K and Atmospheric Pressure
Research Areas Currently no objects available |
Publication Details Author list: Ddamba, W.; Fulele, B.; Nadiye-Tabibiruka, S. Publication year: 2018 Volume number: 8 Issue number: 1 Start page: 13 End page: 25 Number of pages: 13 ISSN: 2167-7042 eISSN: 2167-7069 URL: http://article.sapub.org/10.5923.j.pc.20180801.02.html Languages: English |
Densities (ρ) of pure difuryl methane (DFM), n-pentane or n-hexane or n-heptane or n-octane and those of (DFM + n-pentane or n-hexane or n-heptane or n-octane) binary mixtures over the entire composition range, have been measured at (T = 293.15, 298.15 and 303.15) K and atmospheric pressure. Excess molar volumes Keywords: Difurylmethane, n-alkane, Excess molar volume, Excess partial molar volume, Binary mixtures, Intermolecular interactions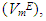 of each binary system were determined and correlated by the Redlich-Kister equation. The
of each binary system were determined and correlated by the Redlich-Kister equation. The 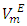 vs x2isothermsfor the (DFM + n-pentane or n-hexane or n-heptane) binary mixtures exhibit a sigmoidal behavior while that for the (DFM + n-octane) binary system positive deviation for
vs x2isothermsfor the (DFM + n-pentane or n-hexane or n-heptane) binary mixtures exhibit a sigmoidal behavior while that for the (DFM + n-octane) binary system positive deviation for 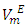 function was observed. The
function was observed. The 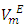 values decreased with temperature increase for each binary systems which suggested a possible structural effects. The
values decreased with temperature increase for each binary systems which suggested a possible structural effects. The 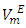 data have been used to derive excess partial molar volumes (
data have been used to derive excess partial molar volumes (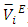 ). Results are discussed in terms of possible geometrical and dispersive intermolecular interactions.
). Results are discussed in terms of possible geometrical and dispersive intermolecular interactions.
Projects
Currently no objects available
Currently no objects available |
Documents
Currently no objects available
